I wrote about BD Intelliport back in January. It’s an impressive system. Others think so, too.
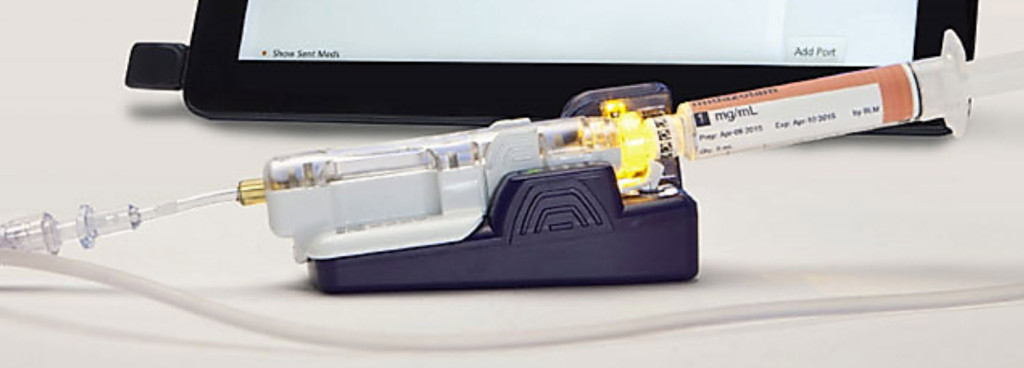
PR Newswire: “Based on its recent analysis of the intravenous drug delivery devices market, Frost & Sullivan recognizes BD with the 2015 North American Frost & Sullivan Award for New Product Innovation. The company’s BD Intelliportâ„¢ System is the industry’s first medication management system designed to overcome long-standing challenges associated with the administration of manual intravenous bolus injections. By offering enhanced automated documentation, dose measurement, drug identification, and real-time allergy alerts to the clinician, the BD Intelliportâ„¢ System is poised to address the most relevant challenges in the intravenous drug delivery market…At the heart of this innovative new system is the BD Intelliportâ„¢ Injection Site, which has a unique barcode reading feature that automatically identifies the medication and concentration contained within a specially encoded syringe as a byproduct of existing clinical workflow. This feature eliminates the need for manual barcode scanning of syringes, enables audible confirmation that the correct drug is about to be given, and facilitates real-time drug allergy checks and alerts. Knowing the exact time each IV injection is given also allows the system to provide accurate and automated re-dosing reminders.”
There’s a lot going on with the BD Intelliport System. The key for me however, is its integration to the EHR. Being able to access clinically relevant information and document in real-time is important.
I would like to see BD incorporate this technology into the BD Cato IV Room System. BD Cato is an impressive workflow management system. It offers bar code scan verification, image-assisted documentation, and gravimetrics. What it doesn’t do is record the volume of medication injected into a CSP in real-time. In all fairness, no one offers such a feature.
One has to wonder whether or not anyone at BD has thought about incorporating the CRISI Medical Systems technology into BD Cato. If not, take the hint. At least consider it for chemotherapy and/or other hazardous drug compounding.
Now for the rest of the story…
I agree with you that this is a very intriguing product. A similar type of solution, by the name DocuSys, was introduced to the market over 15 years ago (http://www.pppmag.com/documents/V5N9/p26.pdf). Like the BD Intelliport System, it used barcode checking in conjunction with a “syringe flow detection system” to accurately measure the volume of IV push medication being administered. This product was picked up by McKesson back in December, 2003, but as far as I can tell it is no longer available (the original http://www.docusys.net webpage is now invalid, and you can buy the http://www.docusys.com URL for $45K, if you are interested).
In additional to the drug administration component of DocuSys it also had a fairly robust Anesthesia Information Management System (AIMS) associated with it. I guess in that regard, DocuSys was doing what you wish the BD Intelliport System did (i.e., integration with an EHR system). I believe the remnants of the DocuSys AIMS product are now part of the Merge Healthcare product set (Merge AIMS), but without any drug administration device.
So, why did the DocuSys syringe-based, IV push medication administration fail and what might that mean for the BD Intelliport System?
Sir Winston Churchill said, “Those who fail to learn from history are doomed to repeat it.
What do I mean by that. Both, DocuSys and the BD Intelliport System had/have a rather elaborate, labor-intensive system to “enable” the syringe administration system to work at the point-of-care. For DocuSys, it was a barcode scannable syringe cradle system that had to be loaded with the correct drug before it could be used. Certainly, anesthesia providers would never do this and the pharmacy departments weren’t all that interested either in “looking for work”. And, this was back before the USP when we didn’t worry as much about beyond-use-dating of syringes prepared in the pharmacy. This is even more complicated today by the fact that many of the syringes available for use (e.g., BD Syringes) are not “FDA cleared for Closed Container Storage” (i.e., they should not be used for drug prepackaging because the potency of the contained drug may be affected).
I find it interesting that marketing spin from Frost & Sullivan which states that the BD Intelliport System “…has a unique barcode reading feature that automatically identifies the medication and concentration contained within a specially encoded syringe as a byproduct of existing clinical workflow.”
The operative phase in that quote is “a byproduct of existing clinical workflow.” What everyone forgot to mention is the BD Intelliport Syringe Labeler. This device is basically like a Codonics Syringe Labeling System on steroids. You must check out the video of this system at the bottom of the BD Intelliport Products webpage (https://www.bd.com/intelliport/products/).
First of all, there is NO WAY that the syringes used in the BD Intelliport are a “…byproduct of clinical workflow”. These syringes are a byproduct of a Rube Goldberg contraption that individually labels the syringe hub of a BD syringe with a barcode label and it also generates a color-coded syringe label. The process is very slow and mechanical as evidenced by the video. They say in the video that the device could be used in the OR, an IV Prep Room in the OR, or in the Pharmacy. There are numerous reasons why any of these are not viable:
(1) There is no way an anesthesiologist is going through this process either in the OR itself or in the OR Area. And, current regulations would not allow anyone else in the OR to prepare medication syringes for administration by anyone other than the person who is going to use the syringe to administer the medication to the patient.
(2) What pharmacy has the staff or resources to prepare syringes using this device and still comply with all of the USP requirements? Besides, the labeling system does not incorporate a mechanism to add a lot number and electronically document the individuals involved in the preparation and checking of syringes prepared in the pharmacy (i.e., no standard pharmacy prepack quality documenation).
(3) Given the recent warning by the FDA that BD syringes should only be used for the immediate drawing up and administration of medications, this would appear to put the damper on any thoughts of preparing syringes in advance using the BD Intelliport Labeling system.
This is clearly a product that was developed WITHOUT the meaningful input of a hospital pharmacist, which is very unfortunate because the drug administration component of the BD Intelliport System is very cool.
The “not so intelligent” kicker for me in the BD Intelliport System video, is how they describe administering syringes containing medications which have been prepared WITHOUT using the BD Intelliport Syringe Labeling System. The user is instructed to draw up the medication in a syringe from a vial and then select the name of the drug contained in the syringe from a “list” on the BD Intelliport Tablet. Yes, you heard me, they don’t even have a way to scan the barcode on the vial used to prepare the syringe, so that you have to rely on selecting a drug name from a list. That is not how any product with this degree of intelligence should work.
I hope someone in BD has already figured this out after performing some clinical trials of the product and, more importantly, trials using the BD Intelliport Labeling System. This needs to change, because if it doesn’t, the value of drug administration component of the BD Intelliport will never be realized.
My suggestions. There are a couple of good OR syringe labeling systems on the market: the Condonics Safe Label System or the Kit Check Anesthesia Check products. Figure out a way to utilize either the barcode on the Condonics system IV syringe label, or the RFID tag on the KC Anesthesia Check syringe label. My preference would be to use the later, Anesthesia Check, by incorporating an RFID reader into the BD Intelliport syringe administration device.
Do this, would allow BD to say that the BD Intelliport System uses an encoded syringe that IS A BYPRODUCT of an existing, efficient, clinical workflow. That’s how it should work.
If BD doesn’t do something to fix the backend (i.e., the BD Intelliport Labeling System), then I’m certain Winston Churchill’s quote will once again be realized again. You don’t hear people talking about DocuSys any more. Will the BD Intelliport System be next…
2024-2-13 What happened to this BD Intelliport Injection system? It seems brilliant to me.
I don’t really know. Way back when, I was very interested in adding it to IV compounding workflow. The company was very receptive to my inquiries and gave me one-on-one access to it. I thought it was great. However, even though I don’t know exactly what happened to it, I believe there was a lack of interest, so it got scrapped.