Can you place items in the primary engineering control (PEC, aka “the hoodâ€)? I get this question from time to time with regards to IV workflow management systems (IVWFMs). Most (all?) IVWFMs currently on the market utilize various hardware components – cameras, stands, scales, tablets, etc. – inside the hood.
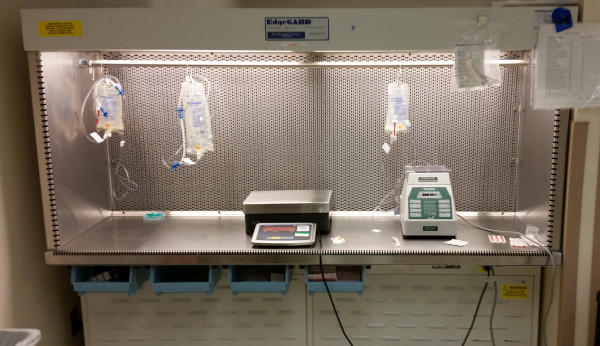
Things may change in the future, but the bottom line is that there is nothing in the current USP General Chapter <797> that directly addresses the use of automation and technology inside the hood.
However, <797> does, in a general way, address the use of items inside the direct compounding area [DCA], which is where compounding equipment is typically found. Nothing found within the DCA can impact first air, and the equipment needs to be cleanable.
USP Chapter <797> states: “After proper introduction into the DCA of supply items required for and limited to the assigned operations, they are so arranged that a clear, uninterrupted path of HEPA-filtered air will bathe all critical sites at all times during the planned procedures. That is, no objects may be placed between the first air from HEPA filters and an exposed critical site.â€
Further guidance may be found in Chapter <797> in a section titled Suggested Standard Operating Procedures (SOPs): “Supplies used in the DCA for the planned procedures are accumulated and then decontaminated by wiping or spraying the outer surface with sterile 70% IPA or removing the outer wrap at the edge of the DCA as the item is introduced into the aseptic work area…. All supply items are arranged in the DCA so as to reduce clutter and provide maximum efficiency and order for the flow of work… After proper introduction into the DCA of supply items required for and limited to the assigned operations, they are so arranged that a clear, uninterrupted path of HEPA-filtered air will bathe all critical sites at all times during the planned procedures.â€
If the items being placed in the hoods meet both requirements, i.e. they do not obstruct HEPA-filtered air and can be properly cleaned, then there shouldn’t be an issue.
To ensure that the items are being used appropriately I recommend that users: 1) perform appropriate surface sampling to ensure sterility, and 2) perform a smoke study. Smoke studies are the only way to ensure that items in the hood are not impeding airflow. Take the time to do one for each hood where hardware is used.