Pulling another article from the notebook archive, penned March 20, 2020.
I have seen the future of IV workflow management systems (IVWFMS). Spoiler alert, EPIC wins. And before people start calling me an Epic fanboy, I should make it clear that I do not like Epic, as a company or a product. I believe healthcare will rue the day they relinquished all their power to a single company.
Those that know me or have read anything I have written in the past decade, know that I am an advocate for technology in the IV room. People are imperfect creatures, they make mistakes. Don’t believe me? Google Emily Jerry death or St Charles rocuronium. That will tell you all you need to know about the dangers associated with injectable medications. Compounded sterile preparations are the most dangerous medications within the four walls of a hospital. Seems logical that such dangers would receive the utmost attention. Inexplicably, they do not. Many reasons are given for ignoring the issues, but it boils down to poor planning and the inability to prioritize in the face of budgetary and political restraints.
Technology, while far from perfect, adds a level of protection to a complex, error-prone, and dangerous process. Adding a little common-sense technology to the IV medication process, like an IVWFMS*, is the quickest and most cost-effective way to improve safety.
Implementing these systems is a no-brainer, but that hasn’t stopped people from ignoring them. The problem has been, at least from my perspective, a complete failure by pharmacy leadership to recognize and prioritize IV room safety and efficiency. Nowhere else but in the IV room can a single mistake result in significant harm or death. Yet the IV room seems to get a fraction of the attention it should. Unfortunately, it often takes a tragic error like those noted above before folks take notice.
With that said, there is some good news. I have witnessed an uptake of IVWFMS in recent years. More hospitals seem to be adding these systems to their workflow. While a welcome trend, the increased numbers don’t appear to be secondary to some altruistic good will or common sense, but rather because of Epic. The monolithic EHR vendor has unwillingly changed the landscape of the IVWFMS market, forever. Big pharmacy technology companies refuse to admit it, but the writing is on the wall. When asked what technology a hospital is using in the IV room, I used to hear “nothing” or “DoseEdge” with an occasional “MedKeeper” thrown in. Now, more often than not, I hear “Dispense Prep”.**
Why the shift? No mystery here, the answer is simple: the barrier to entry is low and the integration within the platform is amazing.
For healthcare systems already using Epic, it is as easy as flipping a switch. The implementation requires a bit of legwork, and some minor equipment, but nothing like that required when implementing a third-party system like DoseEdge, BD Cato, etc. I have been involved with both Epic and third party IVWFMS implementations, there is little comparison in time, energy, effort, and cost. Epic Dispense Prep (EDP) wins in all those areas, easily, every single time.
The ease of EDP implementation is tied directly to the modularity and integration of the overall system. It shares databases, labels, user experience, dashboards, and so on. EDP is already part of the EHR, so it requires no additional contracts, no additional maintenance agreements, no third-party vendor helpdesks, no “integration” within the EHR, no crazy implementation schedule and checklists, no weird upgrade schedule or downtime, and so on.
EDP implementation requires far fewer pharmacy resources than other IVWFMS and has the added benefit of being nearly transparent to pharmacy personnel. Most of the build is handled behind the scenes by dedicated IT resources — the ever present Epic Willow Build Team. Pharmacy resources are kept to a minimum, which decreases impact on the department. Contrast this to something like DoseEdge, which requires a significant investment in time and effort from pharmacy personnel. I can attest from personal experience that the overhead for a third party IVWFMS implementation can be hundreds of hours of dedicated pharmacist time. EDP, on the other hand, requires a fraction of that time. This alone makes it an easy choice for pharmacies strapped for resources, which describes nearly all inpatient pharmacies.
None of this means that EDP is the best IVWMS on the market. Not even close. While it offers full integration across the entire enterprise, barcode scanning, image capture, robust tracking, and is seamlessly tied into the billing system — something I care little about but is a top priority for healthcare systems — it falls short in other areas. As I write this, I can think of at least three products off the top of my head that I believe are better than Dispense Prep. They are more flexible, more feature rich, have better hardware, have better software, and so on. Most even eclipse EDP in the quality of the basics, like image capture. But it doesn’t matter if they are never implemented. The best IVWFMS is the one you are using. While Dispense Prep may not be the best, it is better than nothing. Love the one you’re with, you know?
While not an accident per se, I believe Epic won the battle of IV workflow management systems without trying. Several large IDNS have already converted to Epic, giving them an obvious competitive edge in the IV room. As facilities with Epic gravitate toward Dispense Prep for the reasons outlined above, the market will inevitably begin to contract, forcing third party vendors to compete against one another for a smaller piece of the pie. It may take some time – things always do in healthcare – but companies marketing IVWFMS will feel the pressure. I believe some already have. I have personally witnessed facilities that have uninstalled DoseEdge in favor of EDP, and some that have elected to with Epic over an outside vendor. The pressure is on.
To the IVWFMS out there, I wish you good luck. The long game is not in your favor.
===========================
*Robotics has its place in the IV room. Products continue to get better every year. While it may not be for everyone, I can see use cases where robotics would be a viable option.
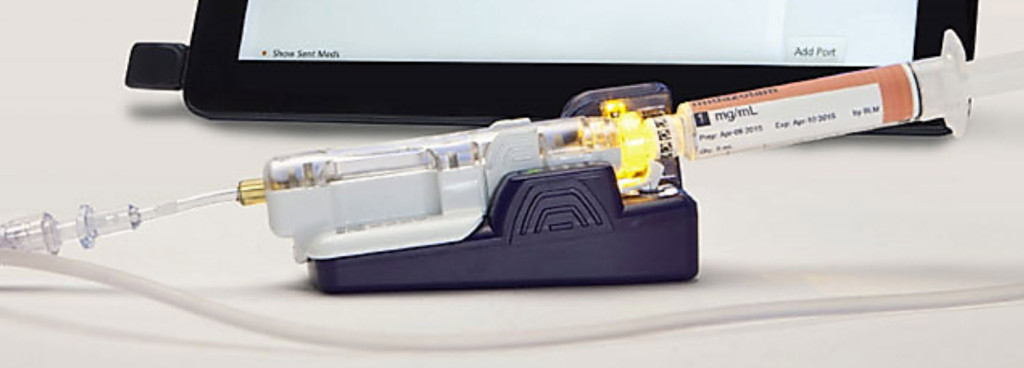
**EPIC Dispense Prep (EDP) is the IVWFMS module inside the Epic EHR System. It is an incredibly well integrated piece of the overall Epic medication distribution model. Dispense Queue [a dashboard of everything waiting to be prepared] → Dispense Prep [capture all data during compounding] → Dispense Check [Pharmacist Review] → Dispense Tracking [track product from pharmacy to bedside]. While I do not care for Epic, in general, one has to admire the vision and design.