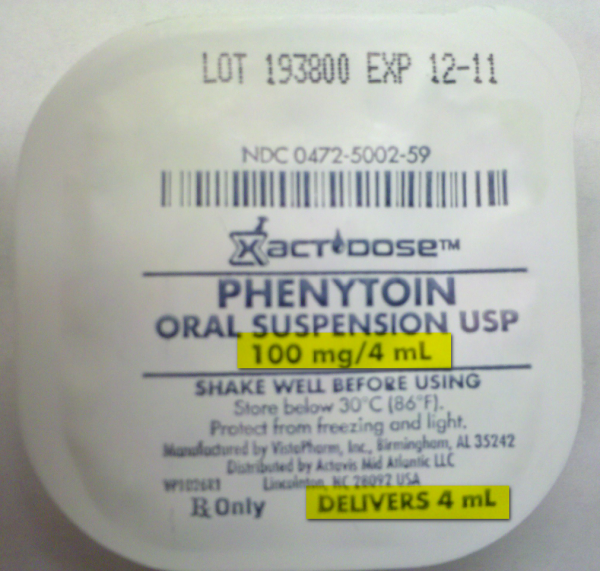
August 12, 2010 issue of the ISMO Medication Safety Alert the issue of : “We have received a number of reports about the labeling of Xactdose unit dose liquid containers from VistaPharm, Inc., of Birmingham, AL. The company recently changed the way the drug concentrations are expressed on their labels. An example is phenytoin oral suspension which went from emphasizing 100 mg/4 mL to listing 125 mg/5 mL. The company rightly notes that the 125 mg/5 mL container delivers 100 mg or 4 mL (due to the heavy liquid consistency of phenytoin suspension), but the message doesn’t necessarily translate to nurses who are confused by the new label and need to give an exact dose. The good news is, we learned last week that VistaPharm is returning to the old style label. That will no doubt lead to less confusion, but nurses should also know not to rinse the residual suspension from the cup. Doing so would approximate as much as a 25% overdose. The company said they expect to release products with revised labeling by the end of the month.â€
This ISMP Alert was perfectly timed because we had confusion over this labeling just a couple of weeks ago. I grabbed one of each label type out of the carousel and snapped a couple of pictures. See below. The top image is of the original labeling, the middle image is the new labeling and the bottom image is the two sitting side by side for comparison.
Liquid unit doses should really be packaged in an oral syringe. Oral syringes are clearly marked to indicate volume, which helps avoid confusion like that caused by unit dosed cup. Another thing I would like to see changed is the use of concentrations like 125mg/5mL and 100mg/4mL. Even though these concentrations are clearly the same, you wouldn’t believe how often this confuses people. Labeling should contain the concentrations in its lowest possible volume, i.e. 25mg/mL, and the dose should be clearly marked, i.e. dose = 100mg = 4mL.


Leave a Reply