Nothing. It says nothing, which leaves things open to interpretation. That’s bad.
Beyond use dating (BUD) in USP <797> is pretty straightforward, but there’s really no language in there describing stock bags.
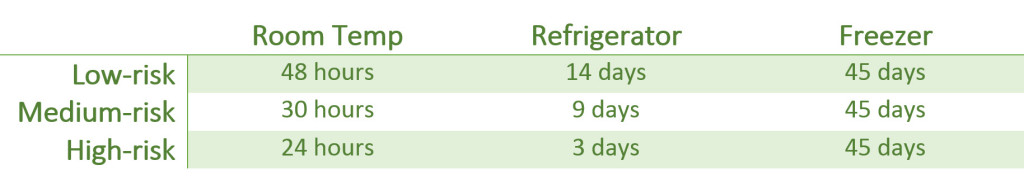
Here are some things to think about. When performing routine compounding, USP <797> states that in the absence of sterility testing, the assigned BUD must not exceed the following:
Here’s USP <797>’s stance on single-dose containers: “Opened or needle-punctured single-dose containers, such as bags, bottles, syringes, and vials of sterile products and CSPs shall be used within 1 hour if opened in worse than ISO Class 5 air quality, and any remaining contents must be discarded. Single-dose vials exposed to ISO Class 5 or cleaner air may be used up to 6 hours after initial needle puncture. Opened single-dose ampuls shall not be stored for any time period. Multiple-dose containers (e.g., vials) are formulated for removal of portions on multiple occasions because they usually contain antimicrobial preservatives. The BUD after initially entering or opening (e.g., needle-punctured) multiple-dose containers is 28 days unless otherwise specified by the manufacturer.”
Is a stock bag a single dose container or something else? I vote for the latter. However, some state boards have taken to treating stock bags as single dose containers, forcing pharmacies to discard unused portions within 6 hours of compounding. Should a stock bag really be considered a single dose container? It’s an interesting question. Without being specifically addressed in writing, state boards can pretty much do as they please. It’s beyond me how the state board can interpret a stock bag differently from any other low-risk or medium-risk level CSP, but they are. At least that’s how things are in California at the moment.
Low-Risk Conditions—
- The CSPs are compounded with aseptic manipulations entirely within ISO Class 5 or better air quality using only sterile ingredients, products, components, and devices.
- The compounding involves only transfer, measuring, and mixing manipulations using not more than three commercially manufactured packages of sterile products and not more than two entries into any one sterile container or package (e.g., bag, vial) of sterile product or administration container/device to prepare the CSP.
- Manipulations are limited to aseptically opening ampuls, penetrating disinfected stoppers on vials with sterile needles and syringes, and transferring sterile liquids in sterile syringes to sterile administration devices, package containers of other sterile products, and containers for storage and dispensing.
I suppose one could argue that low-risk compounding is limited to “not more than two entries into any one sterile contain or package of sterile product or administration container/device to prepare the CSPâ€. Does that still count if one uses a CSTD or other port that prevents re-entry via the stopper? The basic understanding for CSTDs is that they should not be used to extend BUD. However, does this prevent multiple entries into the bag by creating a sealed barrier between the product and the direct compounding area (DCA)? I believe it does, at least in most cases. It is interesting to note that the media-fill test procedure for low-risk compounding includes transferring four 5-mL aliquots of TSA into a single 30-mL vial. Hmm.
A CSP prepared under low-risk conditions receives a 48 hour BUD when stored at controlled room temperature. So if you were to prepare a single ingredient stock bag and leave it in the hood, why not give it 48 hours?
Medium-Risk Conditions—
When CSPs are compounded aseptically under Low-Risk Conditions and one or more of the following conditions exists, such CSPs are at a medium risk of contamination.
- Multiple individual or small doses of sterile products are combined or pooled to prepare a CSP that will be administered either to multiple patients or to one patient on multiple occasions.
- The compounding process includes complex aseptic manipulations other than the single-volume transfer.
- The compounding process requires unusually long duration, such as that required to complete dissolution or homogeneous mixing.
A CSP prepared under medium-risk conditions receives a 30 hour BUD when stored at controlled room temperature. Crud, even compounds prepared in high-risk conditions get 24 hours at controlled room temperature.
If you would have asked me a couple of months ago what BUD to assign to a stock bag, I would have said 30 or 48 hour depending on conditions. However, with news of recent surveys here in California that’s no longer my advice. Until things are sorted out, you’re better off going with six hours. While I do not agree with treating a stock bag as a single-dose container, it is wise to follow current best practice when it comes to the board of pharmacy.
Hopefully the USP committee will address this issue in the next revision of USP <797>.
Leave a Reply