The USP Compounding Expert Committee has published a Notice of Intent to Revise for General Chapter <797> Pharmaceutical Compounding—Sterile Preparations.
I knew this was coming. I’ve talked to several people this year that indicated that revisions to Chapter <797> were imminent, especially with the introduction of USP <800> Hazardous Drugs—Handling in Healthcare Settings.
According the USP notice:
The General Chapter has been under review since 2010 and has been significantly revised to clarify requirements, and reflect stakeholder feedback and learnings since the last revision became official in 2008.
Major revisions of the General Chapter include:
- Reorganization of existing sections and placement of procedural information in boxes
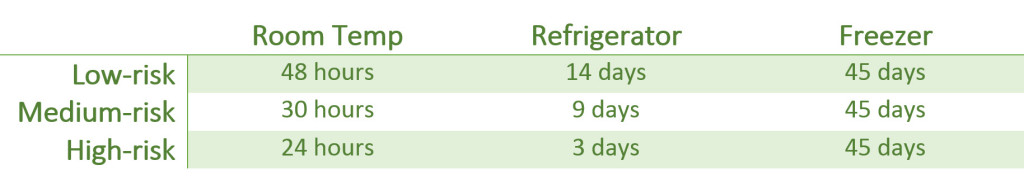
- Collapsing of the three compounded sterile preparation (CSP) microbial risk categories (e.g. low-, medium-, and high-risk) into two categories (Category 1 and 2) distinguished primarily by the conditions under which they are made and the time within which they are used.
- Removal of information on handling hazardous drugs and added cross-references to <800> Hazardous Drugs—Handling in Healthcare Settings
- Introduction of the terminology “in-use time†to refer to the time before which a conventionally manufactured product used to make a CSP must be used after it has been opened or punctured, or a CSP must be used after it has been opened or punctured.
Items #2 and #3 are significant.
Most hospitals do not currently make CSPs that fall into the microbial high-risk category. Altering these categories could have significant impact on acute care pharmacies.
The introduction of USP Chapter <800> Hazardous Drugs – Handling in Healthcare Settings will make any mention of hazardous drugs in the current Chapter <797> obsolete. I suspect that the Compounding Expert Committee will likely remove management of hazardous drugs from Chapter <797> and simply defer to USP <800>, which has yet to be published in anything other than draft form.
I will be spending the next week or so going through the proposed changes to better understand what the USP Committee is thinking. Remember, these revisions aren’t final.
Revisions to General Chapter <797> will be published for public comment in Pharmacopeial Forum (PF) 41(6) [Nov.–Dec. 2015] on November 2, 2015. You can view the proposed revisions with line numbers in advance of publication here [PDF].