It seems as though everyone has chemotherapy on the brain. The National Institute for Occupational Safety and Health (NIOSH) is in the process of updating their Alert on Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. NIOSH already released a new list of hazardous drugs late last year. The U.S. Pharmacopeial Convention (USP) is busy finalizing General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings. And now, ASHP has published updated chemotherapy guidelines.(1)
(more…)
Tag: Pharmacy Practice
-
What practice area benefits most from increased sterile compounding regulation?
I recently sat through a webinar that was recorded during a live symposium at ASHP Midyear in Anaheim on December 8, 2014. The symposium was entitled “Understanding the New Federal Framework for Oversight of Sterile Compounding” (1) and consisted of three separate presentations, one of which was given by Eric Kastango. (2)
(more…) -
Cool Pharmacy Technology – Kiro Oncology System
I’ve recently had conversations with several companies outside the U.S. developing robotic technology for the i.v. room. One of those systems is the Kiro Oncology System. Check the video below.
A couple of things worth noting:
- The system uses dual robotic arms during the compounding process. This is something that is important for the next generation of i.v. room robots. The current crop of i.v. room robots here in the U.S. use a single arm. Think about the inefficiency of one-armed sterile compounding.
- The Kiro Oncology System is self-cleaning. This is a concept that appears to be more popular “in Europe†than it is here in the U.S. Kiro Oncology isn’t the first overseas group I’ve dealt with that is pushing the idea of self-cleaning. None of the U.S. vendors have ever mentioned it.
-
Cleanroom technology for pharmacy – DRUGCAM
DRUGCAM is an interesting piece of pharmacy cleanroom technology. On one hand it falls into the semi-automated systems category because the person using it has to manually manipulate all the components of the sterile compound they’re making. In other words, it’s not a robot. On the other hand DRUGCAM uses some interesting technology and software to automate some of the steps in the process.
DRUGCAM uses multiple cameras(1) to automatically detect the items being used during the compounding process. As the user passes components in front of the cameras, the system automatically identifies them. No bar code scanning required. That’s probably a good thing outside the U.S. as I’ve learned that not all countries require manufacturers to place a bar code on their drug containers. If the system doesn’t recognize the item, the user is notified via visual cues on the screen.
DRUGCAM uses the same technology to automatically detect the volume of fluid pulled into syringes, and also detect when the same syringe is empty following addition of the contents to the final container. I’m not sure how the system determines the correct syringe position, but it’s pretty interesting.
One other thing that makes DRUGCAM unique is that it takes video of the entire compounding process. I’ve mentioned this idea to several vendors over the past few years, but no one really seemed interested in the idea of using video.(2) I think it offers potential advantages over still photos. For one, if something looks weird you can always move forward or back in the compounding process to see what went wrong.
Check the video below. It shows DRUGCAM being used in a glovebox.
DRUGCAM is not currently available in the U.S. If you’d like more information just follow the link to the DRUGCAM website.
—————-
(1) When I saw DRUGCAM at the ASHP Summer Meeting back in June 2013 the engineer told me that the system utilized two cameras, but I can’t find that information on the product website.
(2) Everyone I’ve talk with was concerned about the storage requirements for the video. My brother works for a company that designs security cameras, software, etc. Those companies have been dealing with high-definition video storage for years. -
Drug shortages, whose to blame?
Medscape: “One cause of these shortages, pharmaceutical companies charge, is the amount of time it takes the DEA to approve controlled substance quotas. The DEA has created these quotas for each class of controlled substances and for each manufacturer of drugs containing these agents to prevent their diversion to illegal uses.â€
The drug shortage problem is nothing new. It has become an everyday reality of pharmacy practice. ASHP has established a dedicated website for the problem, and the FDA has gone as far as to create a mobile app to help people track shortage information.
For most people the idea of a drug shortage seems silly, i.e. just make more. The problem is more complicated than that, however. The causes of drug shortages are multifaceted.
(more…) -
Applications to assist with Antimicrobial Stewardship
A couple of days ago I wrote about The California Antimicrobial Stewardship Program Initiative, and how it’s an opportunity for pharmacists to get out and stretch their clinical legs.
Antimicrobial stewardship requires a lot of real-time surveillance and monitoring of patients, labs and cultures, medication use, and so on. There are basically two ways to accomplish this. One is tedious and inefficient, while the other is smart and efficient.
The tedious, inefficient method is the one used by many healthcare facilities. Pharmacies in these facilities simply throw pharmacists at the problem by having them look at a bunch of patients manually every day in search of anomalies. It’s very time consuming. It’s like looking for a crooked needle in a needle stack.
The smart, efficient method involves the use of clinical decision support systems. These systems are connected to several data feeds from other systems throughout the hospital, such as ADT, pharmacy, lab, and so on. The data is aggregated and analyzed against a set of rules designed to find patients with potential problems. These patients are tagged and referred to a pharmacist for follow up, i.e. the pharmacists are only presented with the crooked needles. It’s a much better way to go about things.
There are several systems on the market designed to perform real-time surveillance and clinical decision support. The list below includes many, but is certainly not exhaustive.
-
5 years later, my thoughts on pharmacy practice
I haven’t been a practicing pharmacist in the traditional sense in about five years. I’ve spent the last 19 months as an independent consultant, which has been awesome. Prior to that I was a Product Manager for about two and a half years at a company that dealt in pharmacy automation and technology. Before that I was an IT Pharmacist, which did give me an occasional glimpse of “pharmacy practiceâ€, but overall I figure it’s been at least 5 years since I worked at earnest as a staff pharmacist.
Recently I took a per diem position in a large acute care hospital as a staff pharmacist. I’m completely content being a consultant, and have enjoyed it very much, but I felt that I was losing touch with the daily grind that is pharmacy. I needed to get my hands dirty again and make sure that I wasn’t giving advice to people that was out of touch with reality. I think it’s important for any consultant to be able to relate to the actual problems that they’re being asked to solve. So for the past few months I’ve been staffing about a day a week. Below are some thoughts on what I’ve seen and heard.
(more…) -
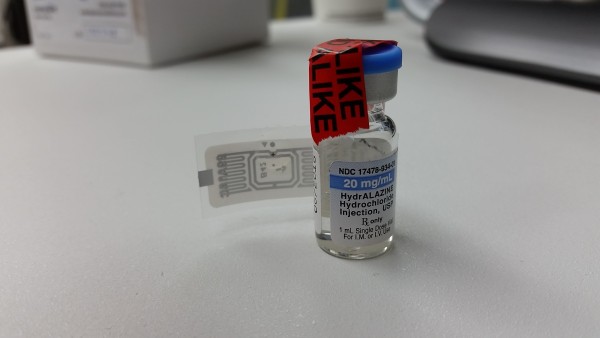
Cool Pharmacy Technology – Intelliguard RFID Solutions from MEPS Real-Time
Last week I spent some time down south in San Diego visiting a couple of hospitals and speaking with the good folks at MEPS Real-Time. My objective for the visit was twofold: 1) see MEPS RFID Solutions in a live environment, and 2) speak with the people at MEPS and get an inside look at their technology. I was able to accomplish both goals.
MEPS Real-Time is a company that specializes in providing RFID solutions for healthcare specifically targeted at acute care pharmacies. Their Intelliguard® RFID Solutions product line currently includes a Kit and Tray Management System, Controlled Temperature Cabinets, and a Vendor Management Inventory (VMI) System.